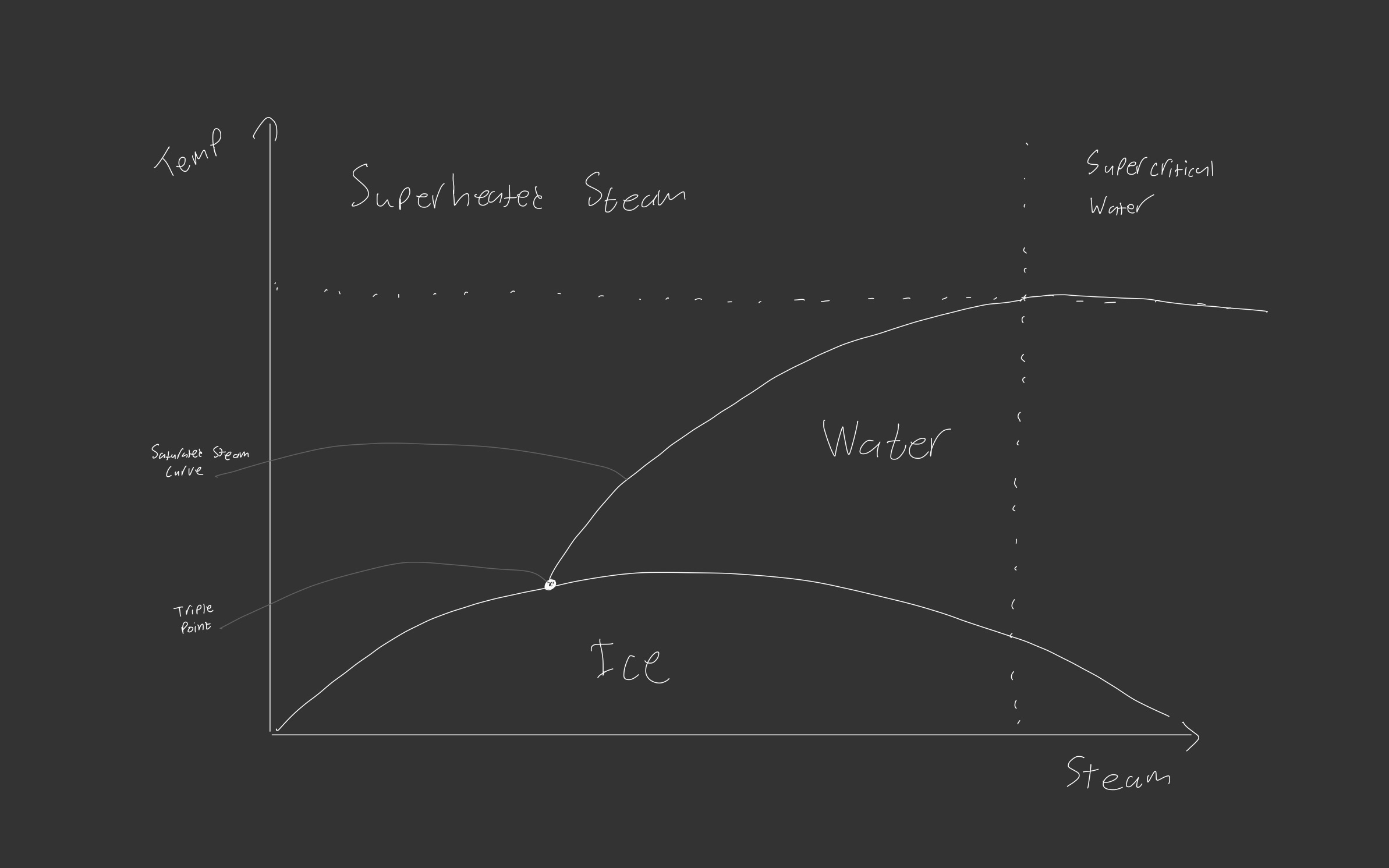
Steam can be in many forms depending on the temperature and pressure it is subjected to.
When moving from water to steam, there is a point where the water starts to become steam, this point where steam is on the edge of turning back into water is called Saturated Steam.
Saturated steam with small temperature changes can change between steam and water, in this state it’s common to get Wet Steam (or Moist Steam), where minute water droplets are carried by steam, this is commonly seen with a kettle where steam turns back into water droplets as it exits the spout. These water droplets can reduce the efficiency of steam sterilisation.
If steam is heated above this saturation point, it becomes Dry steam where there is no droplets and more energy in the steam and harder to condense, requiring more energy to be given off to transition. Although dry steam has more energy, it is harder to condense and as such harder to induce Latent heat transfer into the contact surface.
If steam is heated further it then becomes Superheated Steam, superheated steam has been taken so far beyond its saturation point it is very hard to condense, superheated steam isn’t used in pharma and typically reserved for power generation where high energy density is the name of the game.
Steam is also categorised based on the cleanliness, Plant Steam being unclassified, Clean Steam requiring some level of treatment such as Reverse Osmosis and Pure Steam being the highest level of cleanliness typically requires generation from Water For Injection.